How we successfully treated a patient with refractory relapsed multiple myeloma complicated with TP53 gene variation and central nervous system infiltration using autologous BCMA-CART
How we successfully treated a patient with refractory relapsed multiple myeloma complicated with TP53 gene variation and central nervous system infiltration using autologous BCMA-CART
Multiple myeloma (MM) is a cancer of plasma cells, more common in elderly people and can’t be cured by drugs. In recent years, a large number of new medicines and methods have been applied in MM which has greatly improved the quality of life and overall survival (OS). Currently, chemotherapy, protease inhibitors such as Velcade, vascular and immune modulators such as thalidomide and lenalidomide, and autologous hematopoietic stem cell transplantation are administered in MM, but majority of the patients will finally become resistant to these treatments. Although allogeneic hematopoietic stem cell transplantation may cure MM, most patients can’t accept it due to their old age and organ dysfunction. Once MM become refractory relapse, especially complicated with central nervous system involvement and TP53 gene variation in myeloma cells, the prognosis is extremely poor. It has been reported that the median OS of such patients is less than half a year. In recent years, Nanjing Legend Company in China has developed BCMA-CART, which has achieved good efficacy in the BCMA-positive refractory relapsed MM.
However, the risk of CART therapy is relatively high, especially for those with central nervous system involvement. Here we share our experience in management of a patient with refractory relapsed MM complicated with TP53 gene variation and central nervous system infiltration with autologous BCMA-CART therapy.
Diagnosis and treatment before admission to our hospital
A 66-year-old male patient was diagnosed as MM 4 years ago and admitted to our hospital due to the second relapse for more than 3 months.
In October 2014, the patient felt fatigue and saw the doctor in a famous local hospital. WBC 15.36×109/L, HGB 70 g/L, PLT 40×109/L; BM morphology: 43% of abnormal plasma cells; FCM: expression of CD138, CD56, CD38 and restricted cKappa; Serum and urine immunofixation electrophoresis: IgG-κ monoclonal immunoglobulin. The patient was diagnosed as MM (IgG-κ). Complete remission (CR) was achieved after a cycle of PDD (bortezomib 3.5 mg d1, 4, 8, 11; doxorubicin liposome; dexamethasone 20 mg d1-4, d8-11). Because of lung infection, the second cycle of chemotherapy was adjusted to VD (vincristine and doxorubicin liposome). After his infection was controlled, the PDD was given for another 13 cycles, and thalidomide was applied for half a year.
In June 2018, the patient felt fatigue and obvious numbness in both lower extremities. BM tests and serum immunofixation electrophoresis demonstrated MM relapse. After 5 cycles of VRD (bortezomib 2.2 mg d1, 4, 8, 11; lenalidomide 25 mg QD; dexamethasone 40 mg), MM relapsed again. On January 4, 2019, the patient received a cycle of IRCO (ixazomib 4 mg d1, 8, 15; lenalidomide 25 mg d1-21; cyclophosphamide 50 mg d1-14; dexamethasone 20 mg d1-2, 8-9, 15-16) and then was admitted to our hospital.
Previously, the patient suffered from reflux esophagitis and had smoked for 30 years with averagely 40 cigarettes daily. He had quitted smoking for 4 years before the MM. One of his younger sisters had a history of hemolytic anemia.
Detection and treatment after admission to our hospital
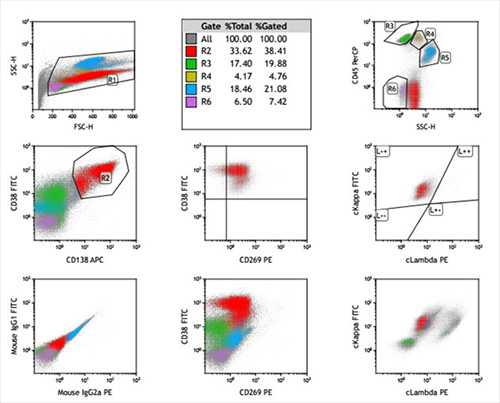
On admission, his heart rate was 107 beats/min, and other vital signs were normal. WBC 2.34×109/L, HGB 70 g/L, PLT 57×109/L; Serum globulin 81.2 g/L, β2 microglobulin 6.81 mg/L; BM morphology: 60% of abnormal plasma cells; FCM: expression of CD269 (BCMA), CD38, CD138, CD56, CD81dim and cytoplasm Igκ; 339 genes relevant to hematologic malignancies were screened by the next-generation sequencing (NGS) and several gene variations were found: TP53 gene function loss; KRAS, DNMT3A and PRDM1 variations. In his cerebrospinal fluid (CSF), WBC was 2×106/L, and 8.51% of abnormal plasma cells were detected by FCM. Infection in both lungs was suggested by CT.
BM morphology in our hospital as following:

The antigen expression of myeloma cells in BM by FCM in our hospital as following:
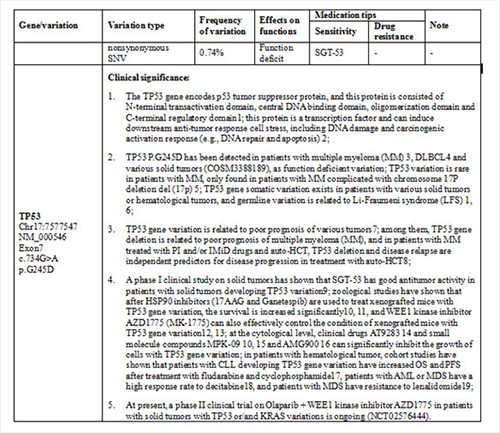
Gene variations of myeloma cells in BM by NGS in our hospital as following:
TP53 p.G245D variation;
KRAS p.Q61H variation;
DNMT3A p.R882C variation;
PRDM1 p.G11Vfs*13 variation.
Because the patient has suffered from MM for more than 4 years, been heavily treated, multiple relapsed and resistant to many drugs including the new protease inhibitor ixazomib, the prognosis is extremely poor. He and his family members requested for BCMA-CART cell therapy and signed the consent form.
Because central nervous system involvement with cancer cells is the main cause of neurotoxicity during CART therapy, chemotherapy drugs were given intrathecally to him for decreasing tumor burden in CSF before CART therapy. When his WBC in CSF was 4×106/L, and abnormal plasma cells were down to 1.65% by FCM, BCMA-CART cell therapy was started. His lymphocytes in peripheral blood (PB) were collected by blood separator (COBE) for BCMA-CART preparation in our hospital (lentivirus encoding murinzed anti-BCMA-CD3ξ-4-1BB CAR from Shanghai Yake Company). Because the patient was in old age with high heart rate and long treatment history, and cyclophosphamide may cause serious cardiotoxicity, the conditioning regimen was with fludarabine 50 mg/day for 3 days only, and then 5.2×105/kg of BCMA-CART cells were infused.
On d4 after BCMA-CART cells infusion, the patient complained that the heart rate was slightly increased. On d7, the heart rate was up to 140 beats/min. On d8, the patient developed fever above 38°C, the body temperature could be temporarily reduced to normal after oral administration of ibuprofen suspension. On d9, the patient developed persistent high fever above 40°C and no response to antipyretic drugs. His heart rate reached 170 beats/min, and dyspnea and obvious hypourocrinia occurred. Abdominal distension, and dry and moist rales in both lungs were detected. It was considered as cytokine release syndrome (CRS) caused by CART cell proliferation and killing tumor cells. In consideration that the patient had long treatment history with old age and high heart rate, it was worried that fatal complications might be occurred by CRS, so methylprednisolone 5mg/kg/d was given, plasma exchange was performed for 3 times, and antibiotics and supportive treatment were applied. Then the patient's body temperature and other vital signs returned to normal on d14.
On d15 after CART cell infusion, no abnormal plasma cells in BM were detected by both morphology and FCM, and serum IgG was significantly decreased. On d27, WBC 2.93×109/L, HGB 88 g/L, PLT 28×109/L, serum creatinine normal, and the patient was discharged. After discharge, his blood cells reached the normal level gradually.
Because the patient’s myeloma cells has TP53 gene variation, he was in high-risk for relapse after CART therapy. Therefore, the patient received oral venetoclax (BCL2 inhibitor) combined with olaparib targeted therapy at home for maintenance. On d92, the patient was evaluated as CR according to the International Myeloma Working Group (IMWG) criteria.
On d43 after CART, the patient developed low-grade fever, cough and expectoration. Moist rales developed in his lungs, and chest CT showed multiple stripy and flaky shadows in the lower lungs, he was diagnosed as pneumonia. The patient was improved and discharged after the treatment with gamma globulin, sulperazone, linezolid and oral voriconazole. Currently, the patient has good quality of life at 4 months after BCMA-CART therapy.
The changes of major parameters after BCMA-CART therapy as following:
1. WBC and lymphocyte (Lym) in PB:
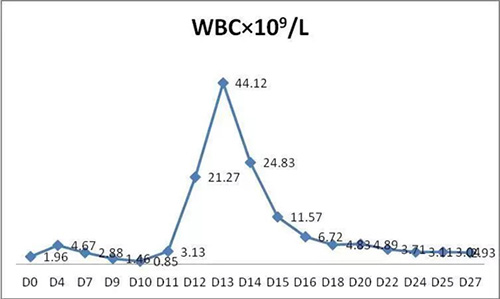
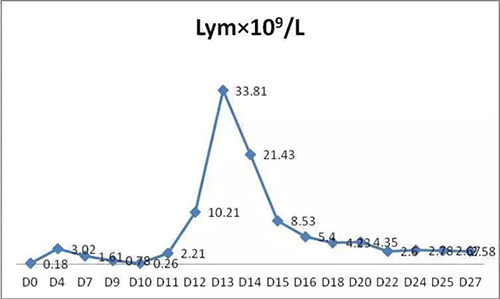
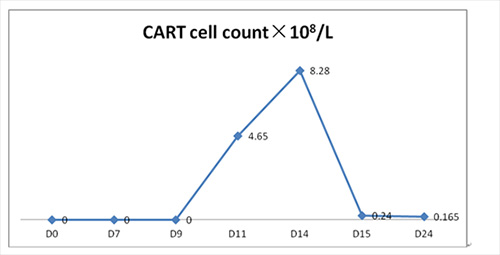
2. CART cell in PB:
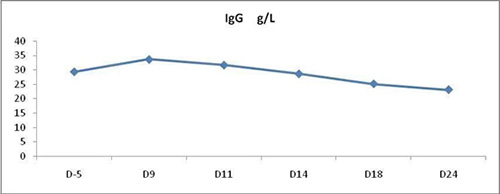
3. IgG in PB:
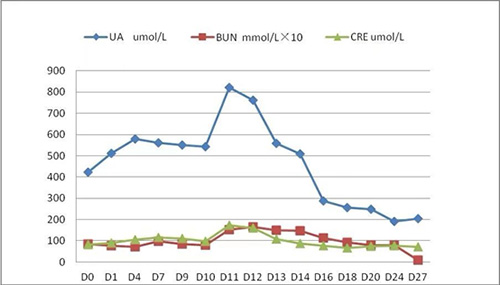
4. Creatinine (CREA), uric acid (UA) and urea nitrogen (BUN) in PB:
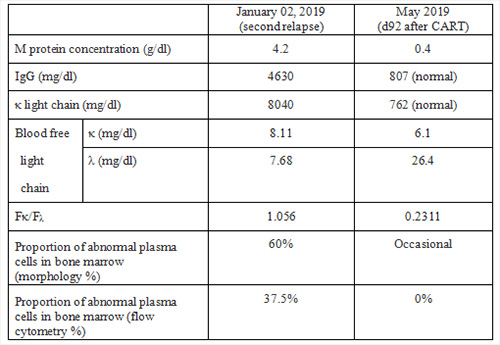
5. Tumor burden before and after CART therapy:
Discussion
MM is an incurable disease. Once relapse, especially complicated with central nervous system infiltration and TP53 gene variation in myeloma cells, the prognosis is extremely poor. It has been reported that the median OS for refractory relapsed MM is less than half a year although new medicines continue to emerge.
The efficacy of BCMA-CART cell therapy in patients with MM has been demonstrated in various clinical trials. This patient underwent multiple cycles of chemotherapy combined with other treatments, but his disease still progressed. New treatment is definitely needed. We successfully treated this tough case with BCMA-CART therapy, which has shown the prospect of BCMA-CART for patients with BCMA-positive MM. The patient experienced severe CRS after CART infusion and was successfully rescued with steroid and plasma exchanged. Finally, he achieved MRD negative CR.
According to previous reports, MM easily relapse again after BCMA-CART, especially in patients with TP53 gene variation. We adopted BCL2 inhibitor combined with olaparib targeted therapy to prevent from relapse. Poly ADP-ribose polymerase (PARP) is an important DNA repair protein in cells and mainly repairs DNA double-strand breaks. Olaparib is a PARP inhibitor and can effectively inhibit DNA damage repair of tumor cells, and in the presence of DNA repair deficiency caused by TP53 variation, it may promote apoptosis of cancer cells with TP53 gene variation, but may increase BCL2 that inhibit apoptosis, so the combination with BCL2 inhibitors may promote tumor cell apoptosis. Long-term observation is required to examine whether such regimen can prolong the remission duration. In addition, due to the expression of CD38 and CD138 on myeloma cells, we may prevent from relapse by sequential CD38-CART and CD138-CART therapies.
In conclusion, BCMA-CART therapy has brought good news to patients with BCMA-positive MM. If the complications can be well controlled, CART therapy can significantly prolong the CR duration and improve the quality of life compared with traditional chemotherapy.
Request an Appointment
Whether you are ready to make an appointment now or have questions for our expert team, we are standing by to help.
Email: international.service@gobroadhealthcare.com Phone: +86 010-83605002/010-83605200 Online Access
















